Autologous Bone Graft Harvesting With the Corex Device
“Not All M.I.S. Harvesting Methods Are Alike”
James F. Marino, M.D., FAAOS (Founder and Medical Consultant) Trinity Orthopedics LLC San Diego, CA
Disclosures:
- The Corex™ Device is manufactured and sold by Trinity Orthopedics LLC
- James Marino is the founder of Trinity Orthopedics LLC
- James Marino is an investor in Trinity Orthopedics LLC
- James Marino is a paid consultant of Trinity Orthopedics LLC
- James Marino is retired from clinical practice, after ~40 years in practice and used the Corex™ device on numerous occasions harvesting from the iliac crest and proximal tibia. He also has harvested with the Corex™ device from various sites in cadaveric demonstrations.
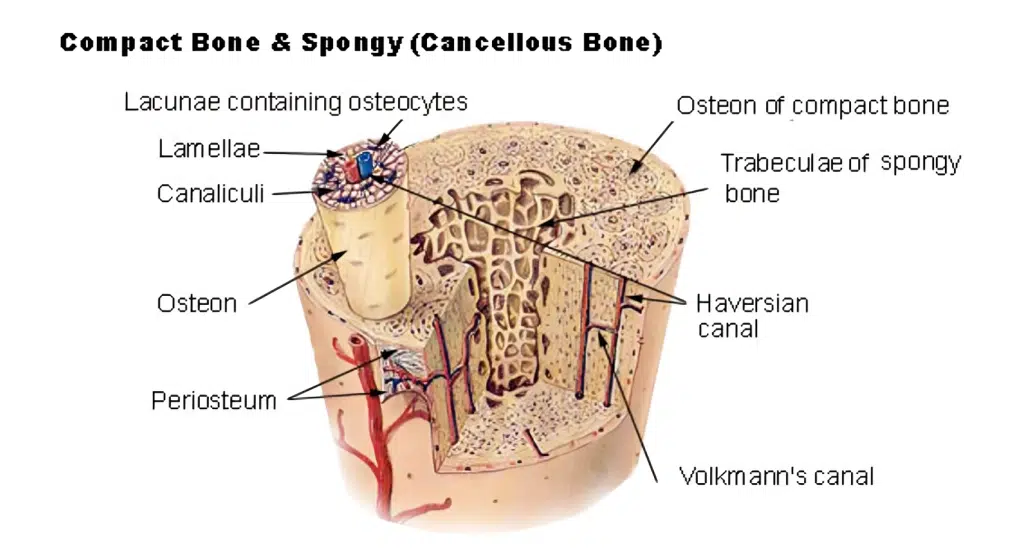
Our Objective – Bone Formation and Subsequent Functional Remodeling at the Host Site
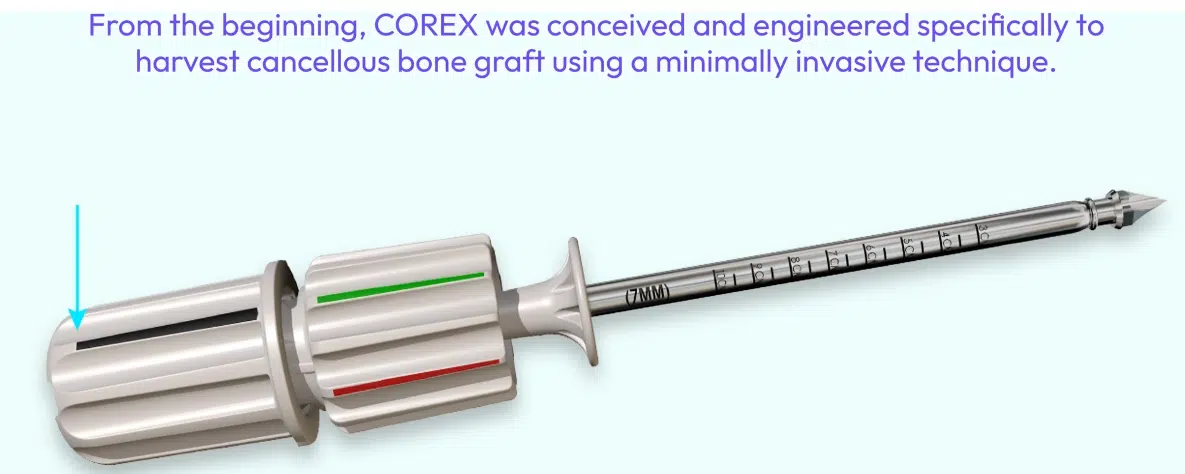
Corex™ Device by Trinity Orthopedics, LLC
Autologous Cancellous Bone Remains the Gold Standard for Bone Graft Applications
- No other bone graft or bone induction material has greater than 100 years of peer-reviewed journal articles demonstrating efficacy in a wide variety of applications (experimental and clinical), with very few authors having any potential conflict of interest.
- No other bone graft or bone induction material is as rapidly incorporated into the host site. “Neovascularization occurs within the graft as early as 2 days after implantation; as it continues, there is a repopulation of the marrow spaces with primitive MSCs of both donor and recipient origin” The Biology of Bone Grafting, Khan et al, J Am Acad Orthop Surg 2005;13:77-86
Complications of Autologous Bone Grafting Primarily Limited to the Donor Site (nonunion excluded)
- Historical donor site complication levels are commonly cited by various parties promoting alternative bone graft material as relevant evidence of the complications of autologous bone grafting. This would be analogous to equating open arthrotomy knee surgery to arthroscopic knee surgery or open laparotomy procedures to laparoscopic procedures.
- MIS techniques in autologous bone graft harvesting have dramatically altered the type and frequency of donor site morbidity, yet many practitioners within the relevant disciplines have a preconceived understanding based upon historical data associated with open harvesting techniques.
- MIS bone graft harvesting is generally percutaneous (or via a very minimal incision), performed through a small cortical defect, and does not involve muscle or tendon stripping.
- Yet not all MIS autologous cancellous bone graft harvesting methods are alike.
Ideally MIS Autologous Bone Harvesting Should:
- Avoid accidental or excessive cortical bone violation utilizing controlled manual or power cortical breaching
- Utilize bone harvesting means that provides for proprioceptive feedback to reduce the risk of unintended cortical breaching Manual rather than powered cancellous bone harvesting
- Provide for multiple redirected passes through a single cortical window
- Provide a cancellous capture mechanism that minimizes distortion of native architecture and preserves cellular content Avoids unnecessary morcellation and suction that dramatically alter the graft
- Avoids cancellous bone desiccation and minimizes handling 6) Employs a procedurally efficient, single patient dedicated device without the need for supplemental instrumentation
Bone Grafting Principles
- Optimize host site for graft incorporation – well vascularized “bleeding” bone surface, debride fibrocartilaginous and devitalized tissue from host bone graft contact surfaces
- Optimize condition of transferred graft for host site integration and bone proliferation – avoid unnecessary cancellous bone morcellation, desiccation, or cellular alteration
- Optimize graft-host contact – place graft in intimate contact with host bone surfaces
- Minimize donor site morbidity – utilize MIS principles for harvesting, percutaneous harvesting if possible
Is bone Grafting Carpentry or Cultivation
- Musculoskeletal Surgeons are often regarded as “carpenters of the body”, but this characterization fails to recognize the vitality of the structures is as or more critical than their configuration.
- Optimizing blood supply and preservation of cell vitality in bone grafting is often under appreciated.
- While allografts and bone graft substitutes are devitalized, one of the distinct advantages of autogenous cancellous bone graft, is the potential to preserve osteo-proliferative cells within the graft.
While “Carpentry” is Important
Cultivation Must Not Be Minimized


Bone Grafting is Cultivation and Not Carpentry Alone


Minimizing Cancellous Morcellation, Desiccation, and Cellular Disruption is Optimization of Autograft Harvesting
- Unnecessary morcellation of harvested cancellous bone distorts architecture thereby delaying revascularization and ultimately requiring “knitting together” fragments at the recipient site
- Autograft harvesters that displace or separate the cellular element from their native positions within the cancellous bone results in diminished cellular viability and differentiation specificity.
- Reassociating the cellular elements with the harvested cancellous bone does not restore osteo-synthetic function of graft cells
Why Consider the Corex™ Bone Graft Harvester
- Single patient use, sterile packaged all in one harvester
- Manual deployment provides for proprioceptive feedback, increasing safety
- Cortical breaching through an integral and patented component that limits depth of penetration
- Cancellous trephine with castellations and a beveled projecting surface to microfracture the perimeter of the cylindrically captured cancellous bone
- Patented capture mechanism, allows for securing the cancellous bone without distorting the architecture or displacing the cellular elements
- Greater than 35,000 clinical uses (since 2007) without a single serious reported complication (i.e. no MAUDE reporting of a serious or life- threatening complication with use)
